In C plants, carbon dioxide enters the Calvin cycle and the first product that results form carbon fixation is 3-phosphoglycerate. C3 plants close their stomata on hot, dry days to limit water loss. In doing this, the concentration of carbon dioxide in the leaf air space falls, which causes the Calvin cycle to slow down
Examples of C3 plants: (Rice, Wheat, Soybean)

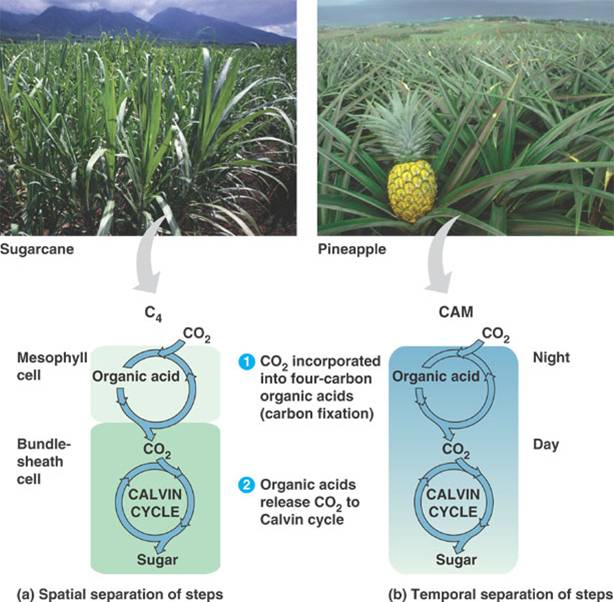
C4 plants open their stomata during the day. In C4 plants, carbon dioxide is added to a 3-carbon compound, known as PEP, with the aid of PEP carboxylase, which has a high affinity for carbon dioxide. The resulting four-carbon compound is formed in the mesophyll cells of a leaf and is transported to the bundlesheath cells tightly packed around the veins inside of a leaf. This compound is then broken down to release the carbon dioxide, creating concentrations high enough that rubisco will accept the carbon dioxide and initiate the Calvin cycle.
CAM plants open their stomata during the night. They perform the reverse of regular plants for photosynthesis. The light reactions are performed during the night while the dark reactions are carried out during the day.
Comparison of C4 and CAM plants: