Sunday, December 18, 2011
Bacterial Transformation (Helped by Michelle Tan)
During the process of transformation, bacteria are "transformed" when they take up DNA from a different strain. As demonstrated in the slide show, the bacteria were transformed into virulence when taking DNA from a virulent strain. This was accomplished through a process known as heat shock since natural transformation is rare occurrence. Lowering the temperature congeals the lipid membrane and stabilizes the negatively charged phosphates found on the lipids and in the DNA plasmid. The temperature imbalance created by the process of heat shock creates an "ionic shield" that allows the plasmid DNA to pass through the adhesion zone in the plasma membrane. In other words, this technique induces the bacteria to take in plasmid DNA and transform by integrating this DNA into their own. The resulting transformed bacteria contained both tetracyline and kanamycin genes (both are antibiotics) and could thrive in the presence of antibiotics.
Thursday, December 8, 2011
3 Beneficial Bacteria (Helped by Michelle Tan)
Lactobacillus
Lactobacillus is a bacterium that can digest lactose. It converts lactose and other sugars into lactic acid. Many studies have shown that lactic acid bacteria have the ability to inhibit the growth of Helicobacter pylori, a pathogen that can cause Type B gastritis, peptic ulcers, and gastric cancer. Lactobacillus has also proven to treat and prevent diarrhea in children. Other potential benefits of this bacterium is the prevention of colon cancer, lowering cholesterol and blood pressure, and inflammation reduction.
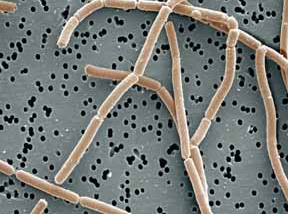
View of Lactobacillus bulgaricus (http://microbewiki.kenyon.edu/index.php/Lactobacillus)
Escherichia Coli
Normally found in the lower intestine of warm-blooded animals, e. coli is known to benefit their hosts by producing vitamin K2 and preventing the establishment of pathogenic bacteria in the intestine. E. Coli strains are relatively harmless but there are some forms that can cause food poisoning in humans. This bacterium is also used in drugs produced to treat and cure illnesses. These drugs include synthetic insulin or antibiotics.
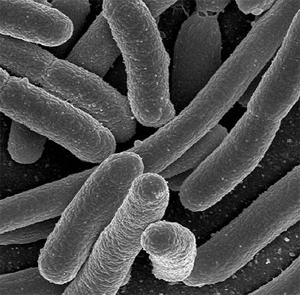
E. Coli Specimen (http://www.freedrinkingwater.com/water-contamination/ecoli-bacteria-removal-water.htm)
Nitrosomonas
Nitrosomonas is an obligate chemolithotrophic bacterium. It is normally found in areas of sewage, soil, fresh water, and marine ecosystems. It functions as a nitrifying bacterium that oxidizes ammonia into nitrate. Nitrosomonas are very useful in the treatment of industrial and sewage waste with the process of bioremediation. Their absence can cause a huge disruption in the nitrogen cycle and the process of carbon fixation.

Lactobacillus is a bacterium that can digest lactose. It converts lactose and other sugars into lactic acid. Many studies have shown that lactic acid bacteria have the ability to inhibit the growth of Helicobacter pylori, a pathogen that can cause Type B gastritis, peptic ulcers, and gastric cancer. Lactobacillus has also proven to treat and prevent diarrhea in children. Other potential benefits of this bacterium is the prevention of colon cancer, lowering cholesterol and blood pressure, and inflammation reduction.

View of Lactobacillus bulgaricus (http://microbewiki.kenyon.edu/index.php/Lactobacillus)
Escherichia Coli
Normally found in the lower intestine of warm-blooded animals, e. coli is known to benefit their hosts by producing vitamin K2 and preventing the establishment of pathogenic bacteria in the intestine. E. Coli strains are relatively harmless but there are some forms that can cause food poisoning in humans. This bacterium is also used in drugs produced to treat and cure illnesses. These drugs include synthetic insulin or antibiotics.

E. Coli Specimen (http://www.freedrinkingwater.com/water-contamination/ecoli-bacteria-removal-water.htm)
Nitrosomonas
Nitrosomonas is an obligate chemolithotrophic bacterium. It is normally found in areas of sewage, soil, fresh water, and marine ecosystems. It functions as a nitrifying bacterium that oxidizes ammonia into nitrate. Nitrosomonas are very useful in the treatment of industrial and sewage waste with the process of bioremediation. Their absence can cause a huge disruption in the nitrogen cycle and the process of carbon fixation.

Stained sample of Nitrosomonas (http://filebox.vt.edu/users/chagedor/biol_4684/Microbes/nitro.html)
Thursday, November 17, 2011
Make a chart of the similarities and differences between cellular respiration and photosynthesis
| Cellular Respiration | Photosynthesis |
| Equation- C6H12O6 + 6O2 ---> 6CO2 + 6H2O + Energy (ATP + Heat) | Equation- 6CO2 + 6H2O ---> C6H12O6 + 6O2 |
| Goes through the processes of glycolysis, Krebs Cycle, and electron transport. | Goes through light dependent reactions and the Calvin Cycle. Broken into photosystems. |
| Catabolic pathway in which oxygen is consumed as a reactant along with organic fuel. Occurs in the mitochondria for eukaryotic cells. | Light energy is converted into chemical energy stored in organic molecules. Occurs in chloroplasts and photosystems. |
| Krebs Cycle involves decomposing the derivative of pyruvate to carbon dioxide. The end products include: 2 ATP, 8 NADH, and 2 FADH2. | The Calvin Cycle involves incorporating CO2 into existing organic compounds through carbon fixation, and these compounds are reduced to form carbohydrates. It uses ATP and NADPH to convert Co2 to sugar. It turns 3 times to fix 3 molecules of CO2 to produce one molecule of glyceraldhyde. |
| Net amount of ATP produced: 36 ATP Glycolysis- 2 ATP, Krebs Cycle- 2 ATP, ETC- 32 ATP, NADH- 2 ATP, FADH2- 2 ATP | 3 types of plants that fix carbon: C3, C4, and CAM. Each has its own mechanism that helps conserv |
| The Electron Transport Chain (ETC) is a collection of molecules embedded in the inner membrane of the mitochondrion. The electron excorts link glycolysis and the Krebs Cycle for oxidative phosphorylation, which uses energy released by the ETC to power ATP synthesis. | Photosystems react to photons reaching the antennae and start the process of photosynthesis. |
Thursday, October 27, 2011
Explain the difference between C3, C4 and CAM plants in terms of their photosynthesis
The difference between C3, C4, amd CAM plants is their process of light and dark reactions. Each type of plant uses an alternative mechanism of carbon fixation that has evolved in hot, arid climates.
In C plants, carbon dioxide enters the Calvin cycle and the first product that results form carbon fixation is 3-phosphoglycerate. C3 plants close their stomata on hot, dry days to limit water loss. In doing this, the concentration of carbon dioxide in the leaf air space falls, which causes the Calvin cycle to slow down
Examples of C3 plants: (Rice, Wheat, Soybean)

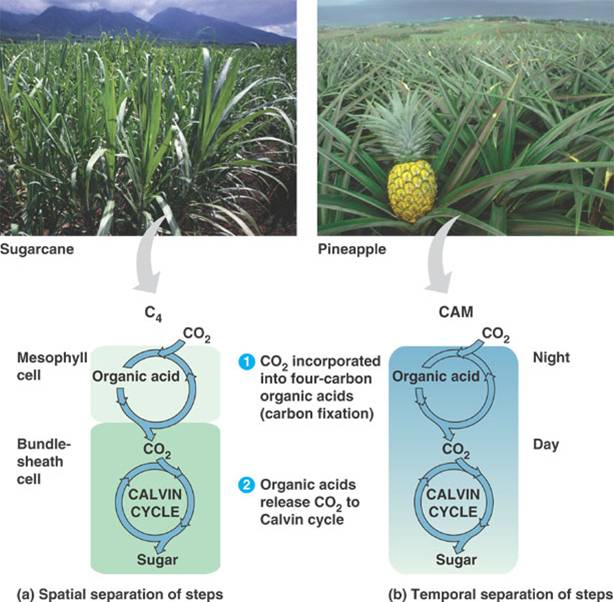
C4 plants open their stomata during the day. In C4 plants, carbon dioxide is added to a 3-carbon compound, known as PEP, with the aid of PEP carboxylase, which has a high affinity for carbon dioxide. The resulting four-carbon compound is formed in the mesophyll cells of a leaf and is transported to the bundlesheath cells tightly packed around the veins inside of a leaf. This compound is then broken down to release the carbon dioxide, creating concentrations high enough that rubisco will accept the carbon dioxide and initiate the Calvin cycle.
CAM plants open their stomata during the night. They perform the reverse of regular plants for photosynthesis. The light reactions are performed during the night while the dark reactions are carried out during the day.
Comparison of C4 and CAM plants:
In C plants, carbon dioxide enters the Calvin cycle and the first product that results form carbon fixation is 3-phosphoglycerate. C3 plants close their stomata on hot, dry days to limit water loss. In doing this, the concentration of carbon dioxide in the leaf air space falls, which causes the Calvin cycle to slow down
Examples of C3 plants: (Rice, Wheat, Soybean)

C4 plants open their stomata during the day. In C4 plants, carbon dioxide is added to a 3-carbon compound, known as PEP, with the aid of PEP carboxylase, which has a high affinity for carbon dioxide. The resulting four-carbon compound is formed in the mesophyll cells of a leaf and is transported to the bundlesheath cells tightly packed around the veins inside of a leaf. This compound is then broken down to release the carbon dioxide, creating concentrations high enough that rubisco will accept the carbon dioxide and initiate the Calvin cycle.
CAM plants open their stomata during the night. They perform the reverse of regular plants for photosynthesis. The light reactions are performed during the night while the dark reactions are carried out during the day.
Comparison of C4 and CAM plants:

Tuesday, October 11, 2011
What I learned about macromolecules and how does the structure of a macromolecule affect its function?
The structure of a polymeric macromolecule consists of repeating units called monomers, the building blocks of a polymer, linked into a chain. The most important factors in the structure of macromolecules is the existence of functional groups. Functional groups are the components that are most commonly involved in chemical reactions. These functional groups can affect the bonds that hold a macromolecule together. For example, the glycosidic linkages that hold together cellulose and starch differ because of a slight difference in their ring structures. The ring form of glucose for starch is in an alpha configuration while cellulose is in a beta configuration. The differences in these two configurations is dependent on the attachment of a hydroxyl group either above of below the plane of the ring. As a result, cellulose and starch serve different purposes (cellulose is a major component of plant cell walls while starch serves as a storage polysaccharide of plants.
These functional groups also affect the polarity of the bonds. The existence of polar or nonpolar bonds ultimately affect a macromolecule's ability to interact with other substances. For example The nonpolar C-H bonds in the hydrocarbon chains of a fatty acid contributes toe the hydrophobic trait of fats. Due to this trait, fats separate from water.
The structure of a macromolecule is very complex. The example shown here is the structure of a protein. The numerous loops and twists contribute to the function of that unique protein. Denaturing a protein causes the twists and loops of the protein to unravel, thus affecting its ability to perform its function. The absence of these traits can basically render a protein useless.

Another example is DNA. Its unique double-helix structure is formed by 2 polynucleotides that spiral around each other around an imaginary axis. The pattern of nucleic acids maps out the process of forming certain proteins in an organism. A mutation in this structure can cause an organism to be incapable of producing an important protein.

These functional groups also affect the polarity of the bonds. The existence of polar or nonpolar bonds ultimately affect a macromolecule's ability to interact with other substances. For example The nonpolar C-H bonds in the hydrocarbon chains of a fatty acid contributes toe the hydrophobic trait of fats. Due to this trait, fats separate from water.
The structure of a macromolecule is very complex. The example shown here is the structure of a protein. The numerous loops and twists contribute to the function of that unique protein. Denaturing a protein causes the twists and loops of the protein to unravel, thus affecting its ability to perform its function. The absence of these traits can basically render a protein useless.

Another example is DNA. Its unique double-helix structure is formed by 2 polynucleotides that spiral around each other around an imaginary axis. The pattern of nucleic acids maps out the process of forming certain proteins in an organism. A mutation in this structure can cause an organism to be incapable of producing an important protein.

Biochemistry Wordle
Biochemistry Wordle Link:
http://www.wordle.net/show/wrdl/4198053/Biochemistry
Vocabulary used: BIOCHEMISTRY, ATOM, MOLECULE, ORGANIC CHEMISTRY, MONOMERS, POLYMERS, MACROMOLECULES, HYDROGEN-BONDING, SOLUTION, SOLUTE, SOLVENT, FUNCTIONAL GROUPS, NONPOLAR, POLAR, AMINO ACIDS, PROTEINS, FATTY-ACIDS, NUCLEIC-ACIDS, NUCLEOTIDES, DNA, RNA, COVALENT BOND, IONIC BOND, HYDROLYSIS, DEHYDRATION, PROTONS, ELECTIONS, NEUTRONS, ACIDS, BASES, VALENCE, MATTER, WATER, SPECIFIC-HEAT
Almost every vocabulary term I used contributes somehow to the structure of some part of life. In my opinion, this chapter really emphasized the theme of structure and function therefore the terminology referring to that theme was the most important. The way a macromolecule is structured ultimately affects its ability to function. This is all applied to things like proteins, nucleic acids, and so on. A tiny difference in a sequence of amino acids can change the purpose and function of a protein entirely. Deeper into the structure of these, we find that many subunits exist. These include atoms, the subatomic particles, and, most importantly, the bonds that hold everything together.
Chemical bonds are what hold everything together and allows certain aspects of life to exist and function properly. For example, hydrogen-bonding, probably one of the most important terms, gives water its unique qualities that contribute to the function of almost every living thing. Without the existence of these bonds, life as we know it would fail to continue to exist.
http://www.wordle.net/show/wrdl/4198053/Biochemistry
Vocabulary used: BIOCHEMISTRY, ATOM, MOLECULE, ORGANIC CHEMISTRY, MONOMERS, POLYMERS, MACROMOLECULES, HYDROGEN-BONDING, SOLUTION, SOLUTE, SOLVENT, FUNCTIONAL GROUPS, NONPOLAR, POLAR, AMINO ACIDS, PROTEINS, FATTY-ACIDS, NUCLEIC-ACIDS, NUCLEOTIDES, DNA, RNA, COVALENT BOND, IONIC BOND, HYDROLYSIS, DEHYDRATION, PROTONS, ELECTIONS, NEUTRONS, ACIDS, BASES, VALENCE, MATTER, WATER, SPECIFIC-HEAT
Almost every vocabulary term I used contributes somehow to the structure of some part of life. In my opinion, this chapter really emphasized the theme of structure and function therefore the terminology referring to that theme was the most important. The way a macromolecule is structured ultimately affects its ability to function. This is all applied to things like proteins, nucleic acids, and so on. A tiny difference in a sequence of amino acids can change the purpose and function of a protein entirely. Deeper into the structure of these, we find that many subunits exist. These include atoms, the subatomic particles, and, most importantly, the bonds that hold everything together.
Chemical bonds are what hold everything together and allows certain aspects of life to exist and function properly. For example, hydrogen-bonding, probably one of the most important terms, gives water its unique qualities that contribute to the function of almost every living thing. Without the existence of these bonds, life as we know it would fail to continue to exist.
Wednesday, September 21, 2011
Vocab Wordle-Ecology Unit
http://www.wordle.net/show/
Terms used: Biodiversity, biophilia, interspecific interactions, competition, predation, carrying-capacity, behavior, innate behavior, classical-conditioning, operant-conditioning, associative learning, learning, imprinting, sensitive period, taxis, kinesis, costs/benefits, trophic levels, energy flow, endangered species, threatened species, and terrestrial/aquatic biomes.
I used a lot of vocabulary related to behavioral ecology because behavior and interactions in an ecosystem are essential components to the functioning of an ecosystem. A species acts the way it does because it is either innate or it is related to the stimuli and other species encountered in an environment. Additionally, I used vocabulary related to the structure of an ecosystem, such as trophic levels and energy flow. Knowing these concepts are important in understanding what links everything in an ecosystem together and keeps it from collapsing. Each trophic level impacts the others directly or indirectly and contributes in keeping energy flowing through in some way.
Terms used: Biodiversity, biophilia, interspecific interactions, competition, predation, carrying-capacity, behavior, innate behavior, classical-conditioning, operant-conditioning, associative learning, learning, imprinting, sensitive period, taxis, kinesis, costs/benefits, trophic levels, energy flow, endangered species, threatened species, and terrestrial/aquatic biomes.
I used a lot of vocabulary related to behavioral ecology because behavior and interactions in an ecosystem are essential components to the functioning of an ecosystem. A species acts the way it does because it is either innate or it is related to the stimuli and other species encountered in an environment. Additionally, I used vocabulary related to the structure of an ecosystem, such as trophic levels and energy flow. Knowing these concepts are important in understanding what links everything in an ecosystem together and keeps it from collapsing. Each trophic level impacts the others directly or indirectly and contributes in keeping energy flowing through in some way.
My Word Cloud
Hi, Ms. Malonek, this is my personal word cloud.
http://www.wordle.net/show/wrdl/4112290/Untitled
http://www.wordle.net/show/wrdl/4112290/Untitled
Subscribe to:
Posts (Atom)